The project
The project
MyPeBS (My Personal Breast Screening) is a unique international project, funded by the European Union. It investigates whether a personalised breast cancer screening could be a better screening option
for women aged 40 to 70.
A need to improve the current
breast screening strategies
Since the late 1980s, organised breast mammography screening programmes have been introduced in more and more countries across Europe, based on the results of 8 large randomised trials. The aim of mammography screening is to detect breast cancer as early as possible, primarily in order to reduce the number of breast cancer deaths but also to reduce the severity of the disease and the use of heavy cancer treatments (and associated anxiety).
These programmes have always used a “one size fits all” strategy whereby women in a target age group (typically between 50-69 years old) are invited for a mammogram (2 incidences bilateral x-ray taken of their breast) every 2 or 3 years. This approach has demonstrated benefits (reduction of breast cancer specific mortality by 20%). However, it also comes with certain side effects, such as false positive findings, overdiagnosis and overtreatment – meaning treatment of indolent cancers (ones that would never have caused problems during a woman’s lifetime because they evolve very slowly), and a small lifetime risk of radiation-induced cancer. Furthermore, the current mammography screening’s sensitivity is not perfect; and the mortality impact not as high as could be expected.
A promising approach to improve mammography screening is personalized, individual risk-based screening.
In the current strategy, all invited women are treated the same. But women are not all the same: each woman has her own individual risk of developing breast cancer, depending on many factors like genetic factors, lifestyle, or hormonal exposure.
Recent scientific advances have largely improved our understanding of breast cancer genetics and other risk factors. We now have accurate risk assessment tools and sufficient knowledge to investigate the advantages of using a new screening approach based on individual risk estimation of breast cancer: this is the goal of MyPeBS.
MyPeBS compares personalised risk-based screening to standard screening
MyPeBS is a European research project that aims to assess the effectiveness and feasibility of personalised breast cancer screening, one that is based on the personal risk of developing breast cancer for each individual woman.
To meet such a goal, at MyPeBS’s core is a multi-centre, international, randomised clinical study that will recruit 85,000 women from Belgium, France, Israel, Italy, the United Kingdom and Spain.
This study will compare the current standard breast screening with a personalised strategy, which screens women at higher risk of breast cancer more often, and women at a lower risk of breast cancer less often.
The goal of this study is to answer a simple, yet fundamental, question: is it better to personalise the method and frequency of breast screening based on a woman’s individual risk?
MyPeBS study scheme
This randomised controlled study will compare two groups of women: a group who will follow the current standard breast screening, to a group who will follow a personalised risk-based screening strategy, which will require a saliva test and an extra visit as compared to the Standard group.
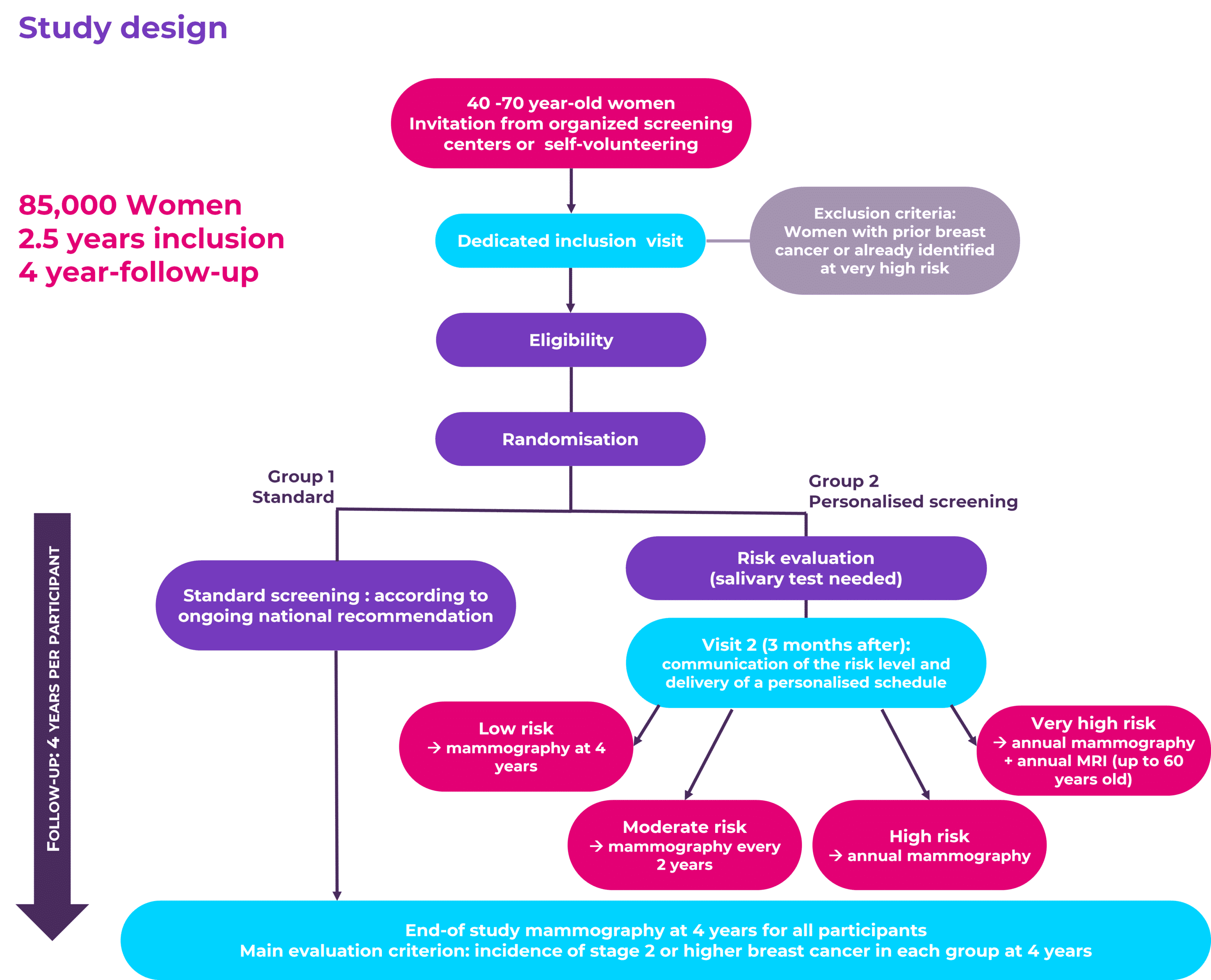
As the personal experience of women is central to the study, MyPeBS will investigate whether the personalised approach is at least equally or maybe more acceptable than the standard one, paying close attention to the potential extra worries for women that may be caused by knowing their individual breast cancer risk.
The project will also evaluate if the economic resources used with a personalised screening strategy justify the results obtained.
Finally, after the results of the study are known, MyPeBS will propose general recommendations for more effective breast cancer screening in Europe.
EU Horizon 2020 funding

MyPeBS has received 12.5 million euros of funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 755394. Horizon 2020 is the financial instrument implementing the Innovation Union, a Europe 2020 flagship initiative aimed at securing Europe’s global competitiveness. By coupling research and innovation, Horizon 2020 is helping to achieve this with its emphasis on excellent science, industrial leadership and tackling societal challenges. The goal is to ensure Europe produces world-class science, removes barriers to innovation and makes it easier for the public and private sectors to work together in delivering innovation.

27 partners
in the MypeBS consortium

8 participating countries

About 1,000 doctors and scientists
involved in the project

85,000 women recruited
in the clinical trial

8 year-duration
for the whole project (2018-2025)

12.5 millions euros of funding
from the Horizon 2020 programme
MyPeBS, a European consortium of leading international breast cancer prevention researchers and experts
The MyPeBS involves 27 partners from 8 different countries (Belgium, United States, France, Israel, Italy, Netherlands, United Kingdom, Spain), including many leading doctors, scientists and prestigious institutions engaged in breast cancer research and prevention. MyPeBS also involves patient’s representatives and advocates from several associations, whose implication from the beginning of the story has been crucial.
MyPeBS is coordinated by Unicancer, a large non-for profit French national federation of hospitals dedicated to oncology and a major European academic sponsor in oncology. The project overall is supervised and controlled by an Executive Committee comprised of key actors of the project, and any decision affecting the governance, composition or financing of the Consortium is taken by a General assembly where each partner is represented.
In addition, the clinical study itself is conducted by a Clinical trial steering committee, and supervised by an independent Ethics and data monitoring committee.
The MyPeBS study is supported by the French Health Insurance and the French National Cancer Institute (INCa), and has been approved by the Cancer Screening Strategic Committee chaired by the French General Director of Health.
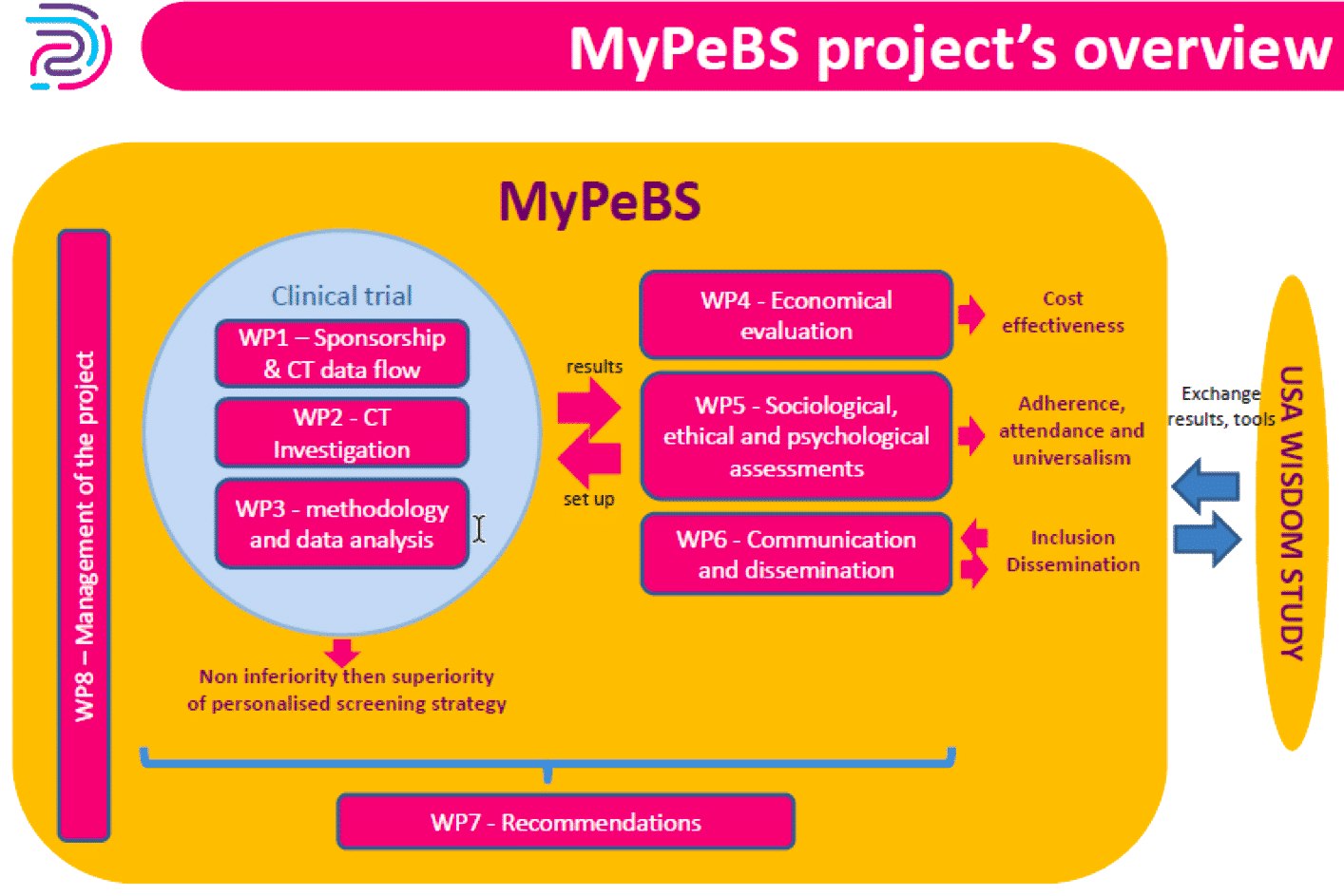
Workpackages
MyPeBS is organized in 8 workpackages :
- WP1: Sponsorship and clinical trial dataflow
- WP2: Clinical trial investigation
- WP3: Methodology and data analysis
- WP4: Economical evaluation
- WP5: Sociological, ethical and psychological assessments
- WP6: Communication and dissemination
- WP7: Recommendations for future Breast Cancer Screening Strategy
- WP8: Project coordination and management